Introduction
This page documents ongoing attempts to produce a rich, variegated green glaze using an iron / phosphorus reaction in borosilicate glaze at cone 6.
Some key takeaways so far:
- A lithium-rich base produces a better green than a soda or potash base
- A small addition of lanthanum oxide appears to ‘brighten’ the effect without changing the color
Iron-Phosphate Glaze
The ‘Blue Hare,’ ‘Hare’s Fur,’ or ‘Floating Blue’ effect is widely-used in pottery studios and commercial glazes.
The effect is observed in saturated iron-rich glazes with the addition of a titanium or phosphorus source. The range of expressed colors varies significantly depending on the base composition of the glaze.
I opted to pursue the iron/phosphate approach, both to build upon existing work and for personal preference. An iron/titanium basis might serve just as well.

Inspiration
In 2023, Clara Giorello published the recipe for a glaze experiment called Selva / Jungle, which aimed to produce the Blue Hare effect. The result was unexpectedly green, much greener than any reactive iron / phosphate glaze I’ve seen. The surface appears predominantly green with tiny gold streaks. On certain test samples, strange isolated blue spots are observed, which implies two different reactions might be occurring.
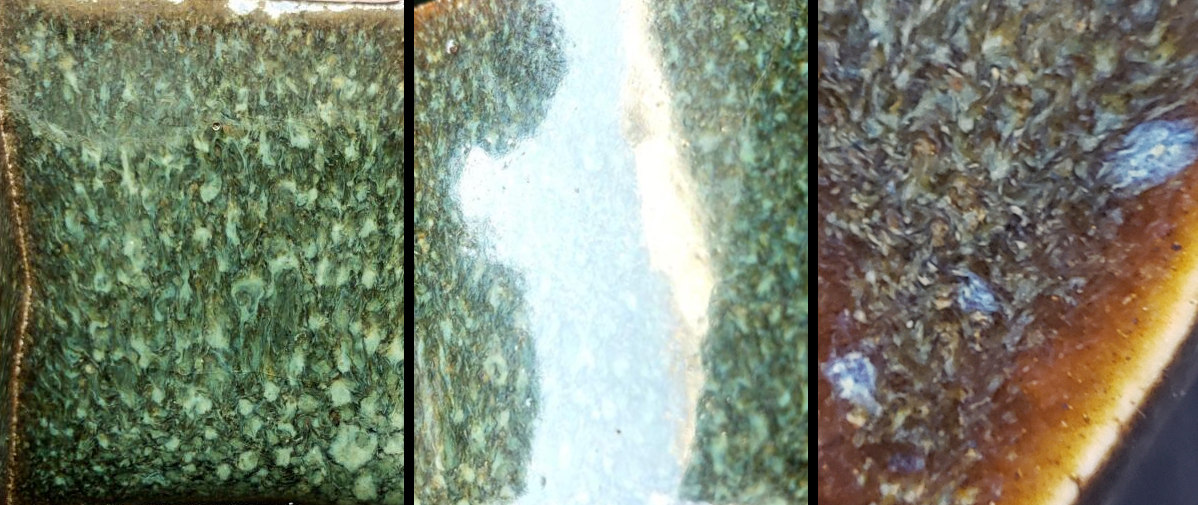
Early Attempts
I began my first attempts to replicate Selva / Jungle without much success - the test tile was amber with only slight signs of blue nucleation points.

I managed to get some variegation by adding rutile (titanium). This new base recipe led to some promising tangents, but nothing was close to the intended effect.
The next attempts involved matching Clara’s instructions as closely as possible:
- Located feldspar and kaolin whose analyses more closely matched ingredients from Argentina
- Used an identical cone 7 kiln firing schedule
- Tried homemade bone ash as my phosphorus source
None of these modifications seemed to offer any improvement, and every test looked more or less like the brown tile pictured above.
Breakthrough #1 - Saturation
The first significant progress toward green coloration involved removing some kaolin from the recipe to better saturate the glass. This almost immediately produced a toothpaste-green when fired at cone 7.

At this point I gave up trying to crack the original recipe, thinking there must be some unknown material or environmental factor that I’d never be able to replicate. Instead it would be more interesting and productive to focus development on new ideas.
Breakthrough #2 - Lithium
Next, I focused on varying primary and secondary fluxes. Early on I established that even small additions of magnesium would turn everything brown. The glaze already contained calcium, sodium, and small amounts of potassium, so the first test involved shuffling ingredients to modify their percentages.
My initial hypothesis was that replacing sodium with potassium would help shift the color towards green. I decided to experiment with lithium as well, since it would help reduce crazing caused by the low kaolin content.

In the test above, the left column is a high-sodium base (Minspar), the middle a high-potassium base (Mahavir feldspar), and the right a potash + spodumene base that replaced much of the sodium with lithium. From top to bottom the whiting was varied.
This test was a little too coarse to draw any hard conclusions, but I was very excited about the low calcium, lithium base (top right) tile. It was faint, but close inspection under light revealed predominantly green and yellow streaks, with a complete absence of blue.
Oddly, this tile also precipitated iron on the edges - the only time I’ve observed this in any test.
Breakthrough #3 - Lanthanum
Some failures later, I hail-mary’d 3% lanthanum oxide into the best lithium-based formula. Very surprisingly, it brightened the initial dark green color to a light army green, without introducing any blue.
To explore this more I tested a line blend, starting from the lithium base (right), and gradually adding lanthanum oxide while removing an equivalent amount of whiting.
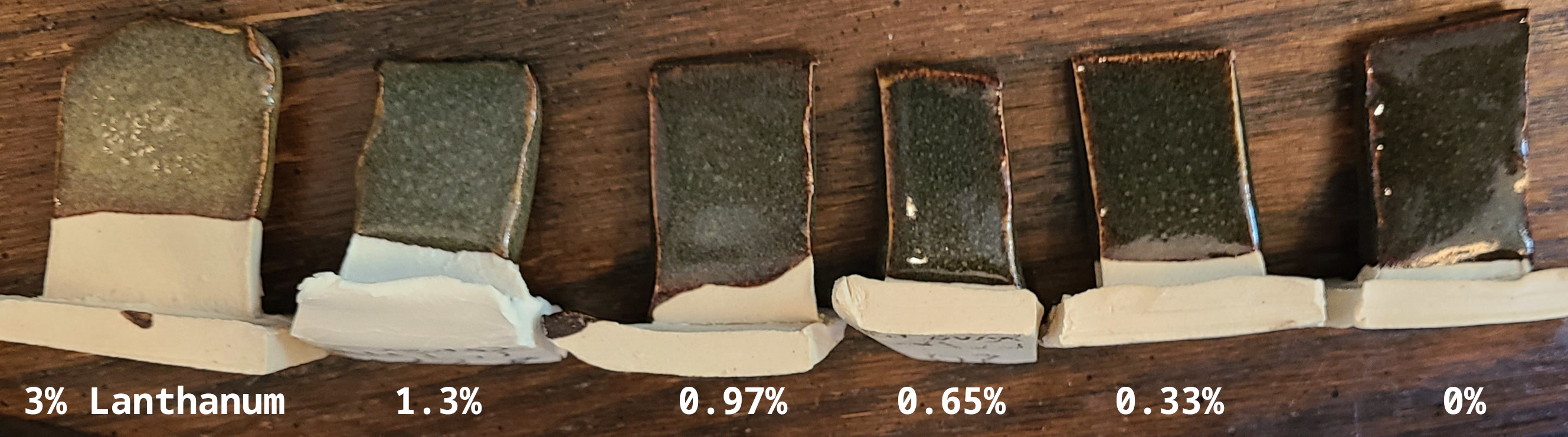
The 1 - 1.3% lanthanum oxide range produced by far the best greens yet.
Checkpoint - v1.7
I was happy enough with the results at this point to start glazing some pieces. This version is published on Glazy.org as ‘Selva / Jungle (USA)’ v1.7.
Pros:
- Decent thermal expansion, fits my porcelain and some stoneware without crazing
- Designed for cone 6, slow cool
Cons:
- Variegation / phase separation is not as pronounced as I’d like
- There’s very little clay in the recipe, so mixing a bucket of it is maybe not a good idea. Future revisions will incorporate more clay and less feldspar

Further experimentation to-do (06/29/2025)
- Confirm theory that lanthanum oxide specifically is brightening the color
- Biaxial blend of iron and phosphorus to try increasing translucency, contrast, and phase separation
- Fine-tune the primary fluxes (lithium, potassium, and sodium) for the best green